FDA opioid safety program fails public
Published 2:00 pm Friday, September 21, 2018
- Investigate TV
The Food and Drug Administration knew for years rogue doctors were wrongly prescribing the most potent opioids on the market by subverting the agency’s oversight program.
Yet the FDA accepted the word of pharmaceutical companies administering the program that they were policing the doctors. But they weren’t and patients died.
More than 4,000 documents, obtained by InvestigateTV through Freedom of Information Act requests, paint a picture of lax oversight and missed opportunities to clamp down on the fentanyl class of opioid prescription abuses.
The records show the FDA knew the dangerous drugs intended only for cancer patients in overwhelming pain were also prescribed by the doctors for non-cancer patients.
The FDA ceded virtually all risk evaluation and mitigation control of the oversight program to the drugmakers of transmucosal immediate-release fentanyl opioids with the narcotic strength 100 times that of morphine.
The drugmakers, in turn, failed to ban doctors with repeated violations from prescribing the drugs, the records show. One doctor continued in the program even after he was indicted by a federal grand jury for health-care fraud and distributing controlled substances.
The oversight program known as TIRF-REM was created by the FDA in 2012 to add an extra level of safety protection for use of fentanyl drugs because of the high risk of abuse, misuse and overdose.
Doctors were required to take an exam about the restricted use of TIFR drugs; patients had to sign a form stating the risks associated with the drugs and that they were intended only for adult cancer patients already tolerant to less powerful opioids for their pain.
Documents and interviews show the FDA for years took a hands-off regulatory approach, relying instead on the drugmakers to enforce the protocols of the safety program.
This lax enforcement had consequences: the majority of patients prescribed the potent fentanyl drugs didn’t qualify and some of them died. But the drugmakers prospered from sales of the high-priced drugs.
“I really don’t know how Big Pharma got involved in a program meant to control Big Pharma,” said Bill Renton, a former agent with the U.S. Drug Enforcement Agency.
Unheeded warnings
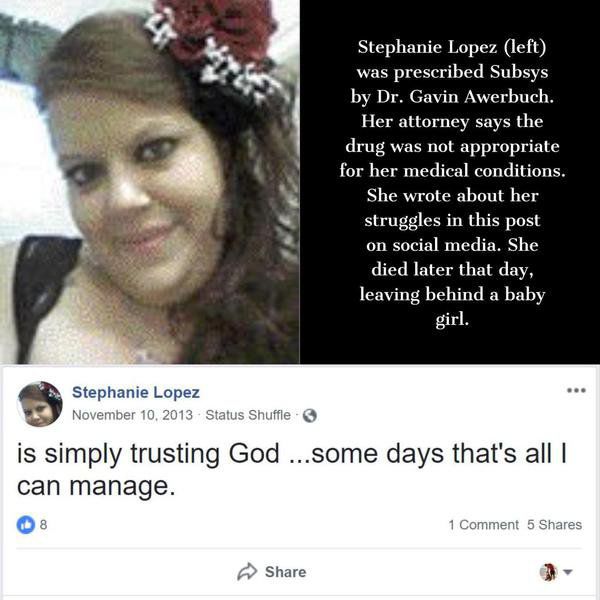
Stephanie Lopez had a litany of serious health ailments: traumatic brain injury, chronic obstructive pulmonary disorder, sleep apnea and complex regional pain syndrome.
What she didn’t have was cancer.
Even so, Dr. Gavin Awerbuch of Saginaw, Mich., prescribed her one of the most potent opioids on the market – the fentanyl spray Subsys, restricted to patients with severe cancer-related pain.
Awerbuch began prescribing Subsys to his patients almost as soon as it hit the market in 2012. The drug industry overseers, who were supposed to watch for abuses among prescribers, knew he was a problem by March 2013, FDA records show.
That month Awerbuch failed to file the required paperwork for 62 patients, meaning those people received fast-acting fentanyl without the signed consent forms stating they understood the risks and were opioid-tolerant cancer patients.
TIRF drug safety protocols require a warning letter if a doctor fails to file five patient forms. According to the records, Awerbuch didn’t receive the first warning until the industry oversight group discovered it was missing more than five dozen forms.
Even after two warning letters and protocol counseling, Awerbuch continued to prescribe TIRF drugs without the proper patient paperwork.
What’s more, the oversight regulators closed Awerbuck’s non-compliance case despite his lacking consent forms from 53 patients.
Less than a month later, Lopez was dead.
“It’s outrageous. It’s very disturbing,” Brian McKeen, a Detroit attorney, said of the lack of action to stop Awerbuch. “These are red flags waving in the wind that any responsible government organization or pharmacy company should be heeding.”
McKeen represents Lopez’s estate and her mother in a malpractice lawsuit filed against Awerbuch. The attorney said Lopez, who long suffered from chronic-pain problems, sought out Awerbuch, who promoted himself as a specialist in chronic pain. He was trained as a neurologist.
Before her death, Awerbuch prescribed Lopez the highly potent opioid drug Subsys. Even if Lopez had cancer, she should never have been on the quick-absorbing fentanyl. Warning labels explicitly state Subsys should not be given to patients with respiratory problems because the drug depresses breathing. Lopez had the chronic lung disease COPD and sleep apnea, McKeen said.
“I don’t think she was aware this was a drug that was not appropriate for her,” McKeen said. “She trusted her physician to select the appropriate drug. There are too many doctors in this country who are injudiciously prescribing dangerous narcotics … resulting in preventable deaths.”
It wasn’t until May 2014 that regulators said they became aware Awerbuch had been arraigned on federal charges of health-care fraud and distribution of controlled substances. Even then, he was not banned from the oversight and safety program.
Two days later, Awerbuch resigned as a TIRF opioids prescriber.
Program oversight
The pharmaceutical companies making the fentanyl drugs serve as the primary communicators with the FDA under the agency’s oversight program.
They also serve as the enforcement arm, making sure doctors and pharmacists who prescribe and dispense TIRF opioids were in compliance with the rules of the safety program.
Today, there are 10 drugs in the program, including six brand-name products: Abstral, Actiq, Fentora, Lazanda, Onsolis and Subsys. All are absorbed through saliva.
But the drug industry’s oversight group’s main source of communications with the FDA centered on annual assessments of the program, including how many patients and doctors were enrolled and other metrics.
A letter from one FDA official shows that after five years of reading these assessments, the agency didn’t have a clear picture of how the oversight and safety program was working.
“We conclude it is not possible to determine whether the overarching goal of the REMS – to mitigate the risk of misuse, abuse, addiction, overdose and serious complications due to medicine errors – is being met,” wrote Dr. Judith A. Racosin, deputy director of safety at the FDA’s Center for Drug Evaluation and Research.
Congressional scrutiny
Multiple data studies show the majority of TIRF patients were not opioid tolerant and did not have cancer and thus should not have received highly potent opioid prescriptions.
This was highlighted at an August FDA public advisory committee hearing that was called after InvestgateTV’s story earlier this year on the McKesson Corp., one of the nation’s largest drug distributions, handling of the TIFR-REM oversight program. U.S. Sen. Bill Cassidy contacted the FDA after watching the report.
“It is only because of your (InvestigateTV’s) inquiry that they agreed to hold this public advisory committee hearing and that is a result of your good investigative reporting,” said Cassidy, a Republican from Louisiana.
At the FDA hearing, the Centers for Medicare Services testified 72 percent of Medicare patients prescribed TIRF drugs did not have a cancer diagnosis in the same year. A study by the drugmakers themselves concluded 42 percent of TIRF patients were not opioid tolerant.
“Are prescribers lying on the forms? Why are we having this disconnect? And if we have a disconnect how can we be concluding that the REMS (oversight) program is operating as intended?” asked Steven Meisel, a patient safety expert at the Institute for HealthCare Improvement based in Minnesota.
The FDA responded that it takes a hands-off approach.
“We don’t make certain the patients are opioid tolerant,” said FDA representative Elizabeth Kilgore. “There’s no information as to whether they have cancer or not.”
Critics of the program, such as New York lawyer Richard J. Hollawell, compare the agency’s oversight and safety program to the fox watching the henhouse.
“The FDA conceded all control to the drug industry and it’s disgusting,” he said.
Hollawell represents parents who lost their 32-year-old daughter to a Subsys overdose and a New Jersey mother who became addicted to the drug. Neither had cancer. Neither was protected by the oversight program.
“The FDA holds itself out that it is the gatekeeper for the protection of the consumer. The FDA is doing the opposite,” Hollawell said. “They are allowing the industry to dictate how these drugs get to consumers and it’s all in the interest of the drug industry.”
Changed attitude
In 2017, the FDA finally flexed its muscle with TIRF drugs.
It instructed the pharmaceutical industry oversight group to clearly state they are intended only for cancer patients and “are not to be used for acute, post operative, emergency room or dental pain,” FDA records show.
By then, the most popular TIRF drug, Subsys, had left a trail of dead and injured patients and indictments against doctors and company insiders. The drug had been routinely prescribed to patients without cancer
At the end of last month’s hearing, FDA committee members urged more education for prescribers beyond the 30-minutes every two years estimated by McKesson. FDA has instructed drugmakers to analyze outcomes, such as death of non-opioid tolerant patients taking TIRF drugs.
It all comes too late for those patients who lost their lives.
Within six months of Stephanie Lopez’s death, federal investigators had zeroed in on Awerbuch, her Michigan doctor.
He was arrested in May 2014 on charges of health-care fraud and illegally prescribing controlled substances. He eventually pleaded guilty and in February was sentenced to 32 months in prison.
Though he has sued Awerbuch for Lopez’s death, attorney McKeen said the FDA is not without blame because of its indifferent oversight.
“It seems like they were acting like Dr. Awerbuch’s enablers,” he said. “It’s very, very concerning.”
On Nov. 10, 2013, Lopez posted on her Facebook page, “simply trusting God …some days that’s all I can manage.”
She laid down for a nap. She never woke up.
“Unfortunately, Dr. Awerbuch did not put her interests first,” McKeen said. “As a consequence, Stephanie lost her life.”
She was just 30.
InvestigateTV is produced by Raycom Media, Inc., parent company of CNHI.